The surfactants used in many Lion products form a variety of aggregates within said products, such as micelles, vesicles and emulsion droplets (Figure 1). In micelles, the surfactant’s hydrophilic groups are positioned on the outside, on the interface with water, while the hydrophobic alkyl chains form the core. Vesicles are lamellar spherical particles in which the surfactant molecules form a bilayer membrane. In an emulsion droplet, surfactant molecules adsorb to the surface of a droplet of oil or other water-insoluble ingredient to form a granular aggregate.
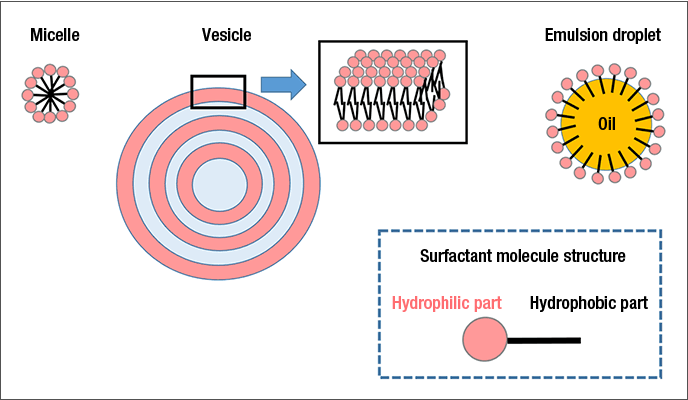
Surfactant aggregates can envelop the water-insoluble active ingredients of products by adsorbing them to their hydrophobic components. Such behavior is deeply connected to product functionality. To enhance product functions, we utilize the latest analytic methods (Figure 2), seek to reinforce technologies for analyzing the dispersion state and detailed interior structures of aggregates, and thereby further develop technologies for controlling aggregate functions.

Figure 2. Equipment Used to Analyze Aggregate Structures and Functions
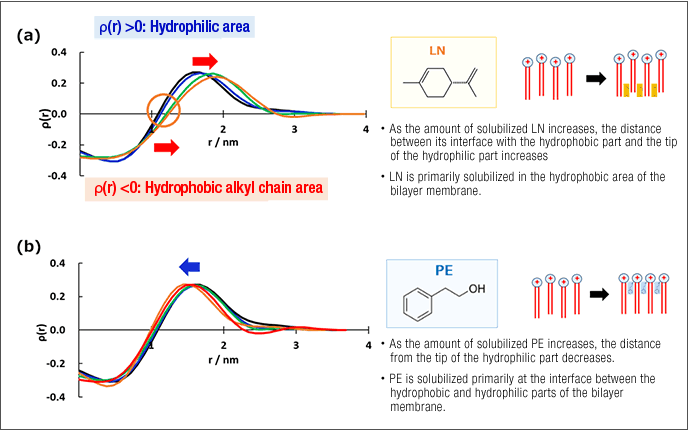
Many fragrance ingredients are solubilized by adsorbing to the hydrophobic components of aggregates. The function sought from fabric softeners is twofold: improving the texture of washed items and imparting a pleasant fragrance. Vesicles formed by cationic dialkyl surfactants are used to this end in fabric softeners. Many fragrance ingredients can be enveloped by vesicle bilayer membranes so that the fragrance is imparted when the vesicles adsorb to the fabric surface. To control the amount of solubilized fragrance ingredients, it is important to understand the structure of the aggregates that envelop them. Using small-angle X-ray scattering, we have been able to determine in detail the physical position of various types of fragrance ingredients within the aggregated that envelop them (Figure 3).1
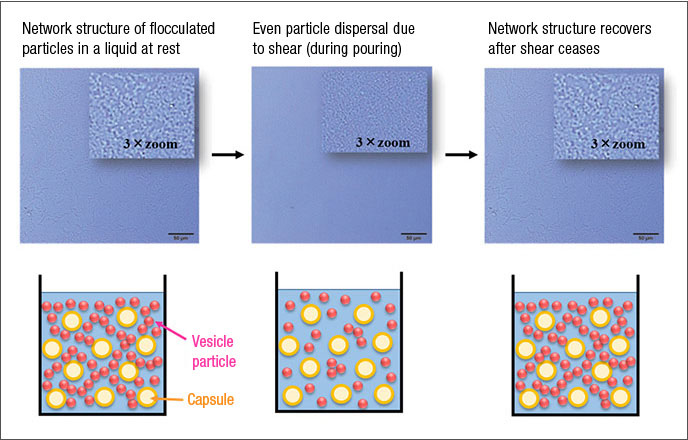
In recent years, consumers have increasingly demanded fabric softeners with long-lasting fragrances, leading to the use of microscopic fragrance capsules. These fragrance capsules have low specific gravity, causing them to rapidly separate and float to the top of a vesicle dispersion liquid, such as a fabric softener . Preventing separation requires technologies to control the dispersion state of such liquids so that the vesicle particles align in a network, forming a structure throughout the liquid. By adding a particular type of polymer to the mixture, we have succeeded in creating a reversable vesicle particle dispersion state in which network structures form when the liquid is at rest, preventing fragrance capsules from floating upward, while allowing these structures to break when the liquid is poured for better flow (Figure 4). We have not only utilized this mechanism, by which these dispersed particles form a solution structure, but been able to determine in detail how it functions.2
1) E.Ohnishi, et al., "SAXSexcites", International SAXS Symposium 2017, 26 to 26 Sep., Graz, Austria
2) A. Miyajima, et al., Journal of Oleo Science , 68, (9) 837-845 (2019)

Rinse-free washing technology incorporated into Acron Smart Care, a laundry detergent (for delicates) with Fabric-softening agents a built-in softening agent
Applying Biointerface Research to Maximize Active Ingredient Effectiveness

Using Aggregate Control Technology to Enhance Product Functions
Related Information