The Three Elements of Infection: Pathogens
We interviewed Prof. Shigeru Morikawa*, a leading researcher on viruses and infectious diseases and on the risk analysis of zoonosis in Japan.
These interviews were conducted in May 2021 by our research fellow, Dr. Murakoshi.
*The professors’ profile can be found at the bottom of this page.
Murakoshi: Today’s topic is COVID-19, the disease caused by the novel coronavirus, an emerging viral infectious disease that falls under the zoonosis*1 category. Could you please explain the basic concepts, ideas and definition of zoonosis to the readers of this journal?
Morikawa: “Zoonosis” comes from the Greek words
“zoon” (animal) and “nosos” (disease). Since humans and animals interact with each other in many
ways, pathogens can be transmitted from animal to human, human to animal, or animal to animal
across species. The numerous emerging infectious diseases that have arisen since around 1970 are
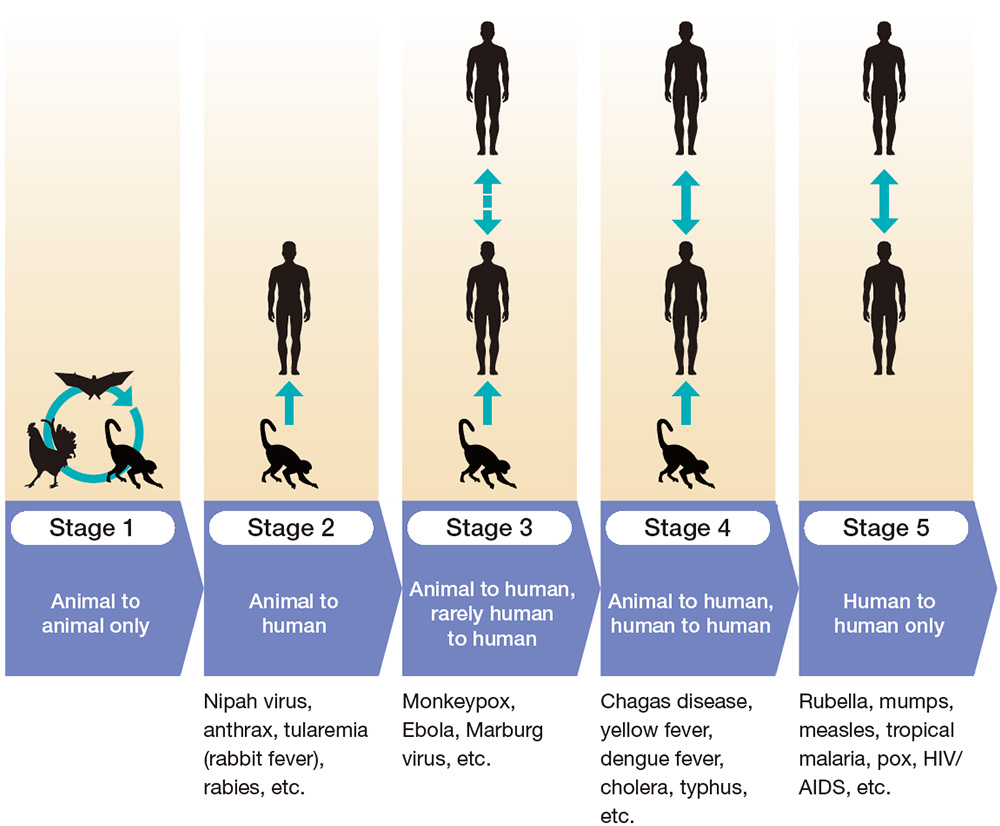
largely zoonoses. Zoonotic pathogens go through five stages of infectious potential to humans
(Figure 1). In Stage 1, the disease is transmitted between animals and is not infectious to
humans. As the disease moves to Stage 2, it becomes infectious from animals to humans, and in
Stage 3, it becomes infectious from animals to humans and, in rare cases, from humans to humans.
When a disease moves to Stage 4, it can be transmitted from animals to humans, and human to
human infection becomes common. In Stage 5, the disease is only transmitted between humans, and
no longer transmitted from animals. Diseases such as measles, mumps and rubella are all
originally from animals and are now in Stage 5, but nowadays, no one calls these diseases
zoonoses.
When new zoonotic viral diseases occur, most are contained at Stage 2—that is, before they can
be transmitted between humans. Infectious diseases in Stage 2 and Stage 3 often have a very high
mortality rate. Infections with a high mortality rate usually do not reach Stage 5.
Murakoshi: Why don’t diseases with high mortality rates reach Stage 5?
Morikawa: It is widely accepted that diseases with high mortality rates do not reach Stage 5 because patients can be isolated and human-to-human transmission can be prevented. In addition, infections that transmit via blood and bodily fluids are unlikely to lead to pandemics. It’s almost always respiratory infections that cause pandemics, such as influenza and now COVID-19.
Murakoshi: SARS and MERS are also respiratory infections caused by coronaviruses that are very similar to COVID-19. Why did neither of these lead to a widespread pandemic?
Morikawa: Although SARS, which has been traced to the horseshoe bat, has a higher severity and mortality rate than COVID-19, it is rarely transmitted from human to human before the onset of symptoms. Most transmission has been within hospitals, from already-hospitalized patients, so it is possible to contain it within hospitals. MERS, which is believed to have originated in dromedary camels that got the virus from bats, also has high severity and mortality. However, because the virus mainly replicates in the lower respiratory tract and causes symptoms in the lungs, thus the infectious particles in coughs or exhalation is limited compared to SARS and COVID-19, so it is not easily transmitted from human to human. COVID-19 is different because there are many asymptomatic cases, and also because transmission can occur even before any symptoms show, so the disease has been able to spread much further than other coronaviruses.
Murakoshi: Why are so many new infectious diseases emerging?
Morikawa: Currently, there are about 1,000 viruses
known to infect humans and cause disease. However, it is estimated that there are about 1.67
million unknown viruses that can infect birds and mammals, and that 600,000 to 800,000 of these
viruses have the potential to infect humans.*2 That means that we know about less
than 1% of all
the viruses in existence.
There’s probably an inexhaustible supply of unknown viruses that have mutated to be
transmissible to humans from animals. I believe that there have likely been quite a few cases of
such viral infections that have been contained before they were well understood, and never
reached pandemic levels.
The genome sequence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is
the causative virus of COVID-19, is said to be similar to that of a virus from the horseshoe bat
family. However, the portion of the sequence encoding the spike protein, which is important in
human infection, is similar to the genome sequence of a coronavirus found in pangolins. Since
coronaviruses are very prone to genetic recombination, the most likely theory for the origin of
SARS-CoV-2 is that the coronaviruses of bats and pangolins recombined, resulting in a virus that
is transmissible to humans.
There is a risk that as human-to-human infections spread, other animals may get infected,
resulting in further mutations, and the emergence of viruses that are more likely to cause
disease, so research into this area is urgently needed. At the very least, I think it would be
valuable to conduct surveillance*3 on pathogens in animals that live in close daily
proximity to
us—like cats and dogs—to prevent the spread of viruses that could cause illness in such animals
and be transmitted to humans.
Murakoshi: That concept—that doctors and veterinarians should work together to prevent the spread of such infectious diseases—is the core of One Health,*4 which you advocate.
Morikawa: Exactly. It’s the idea that the health of people, animals and the environment are fundamentally interconnected. Recently, agriculture has also come to be considered an aspect of One Health. In Asia, antibiotics are often used in pesticides and fertilizers, which creates drug-resistant bacteria in the environment. When these drug-resistant bacteria infect humans or animals, there is a risk that antibiotics will not work and treatment will be difficult, even though the infections caused by these bacteria have not been treated with antibiotics in the past. The One Health approach points to the need to adopt a broader perspective that goes beyond humans and animals and includes the environment as well.
Murakoshi: What are some other recent examples where a virus was only discovered after a large number of people became ill?
Morikawa: SFTS,*5 caused by the
Bunyavirales virus,
was identified after a series of cases of an often fatal disease of unknown cause occurred in
China. Infection occurs when a tick carrying the virus sucks blood from a human or animal. Deer
and wild boar that came to eat crops carried ticks into areas of human habitation, and humans,
dogs and cats were, in turn, bitten by the ticks, allowing the virus to circulate near and
amongst humans. In Japan, there have been cases of infection every year since it was first
reported in January 2013, mainly in western Japan, and more than 70 people have died.
In the past, most new occurrences of infectious disease emerged in Africa, but recently there
has been an increase in those originating in Asia, most likely due to increased contact between
humans and wildlife. In these areas, live wild animals are sold at markets, which is also very
risky. It’s important to avoid close contact with wildlife.
Murakoshi: Are there any infectious diseases specific to Asian countries that we should be aware of?
Morikawa: Yes, rabies. In Japan, it has been eradicated, so it’s not on many people’s radar, but when there is a case of infection, the mortality rate is almost 100%. It is most common in Africa and Asia, most of all in India, but there are still about 50,000 cases per year worldwide. Most cases are caused by bites from rabid dogs.
Murakoshi: I see. We need to be careful of not only wild animals, but also the animals we live with. Lastly, please share your closing thoughts.
Morikawa: I believe that infectious diseases that cause large-scale pandemics, such as COVID-19, and small-scale but highly deadly outbreaks of infectious diseases, such as SFTS, will continue to emerge. When we encounter an illness for which the cause is unknown, I think that we should suspect viral infection. By doing so, we can discover new viruses and, by studying them, be better prepared in case of an outbreak. If you think you might have come into contact with such a virus, please inform the National Institute of Infectious Diseases.
Murakoshi: It’s definitely important to provide such information. Thank you very much for sharing so much valuable information with us.
*1Zoonosis: Defined by the World Health Organization as “any disease or infection that is naturally transmissible from vertebrate animals to humans.”
*2 Carroll D et al. (2018) The Global Virome Project. Science 359: 872-874
*3Infectious disease surveillance refers to the systematic investigation and analysis of outbreaks of infectious diseases in order to develop appropriate infection control measures and help prevent the spread of infectious diseases.
*4One Health: An approach that entails taking animals and the environment into consideration when protecting human health. In recent years, the emergence of drug-resistant bacteria due to the use of antimicrobial agents in a wide range of fields, including livestock farming, and their potential to cause infectious diseases in humans has become an issue, which One Health seeks to help address.
*5Severe fever with thrombocytopenia syndrome (SFTS): A tick-borne disease that causes fever, nausea, vomiting, abdominal pain, diarrhea and bloody bowel discharge, sometimes accompanied by myalgia, neurological symptoms, lymphadenopathy and bleeding. The disease has a mortality rate of 10%–30%. In 2021, cases were confirmed for the first time in Shizuoka and Chiba prefectures. A key precaution to take is to minimize skin exposure when doing outdoor activities to avoid tick bites by, for example, wearing long sleeves, long pants, shoes that completely cover the feet, hats and gloves, and wrapping a towel around the neck. Light-colored clothing makes it easier to visually identify ticks.
Shigeru Morikawa,D.V.M., Ph.D.
Professor, Department of Microbiology, Faculty of Veterinary Medicine, Okayama University of Science

Prof. Morikawa is a graduate of the University of Tokyo Faculty of Agriculture Department of
Veterinary Medical Science, earning his undergraduate degree in 1981, his master’s degree
from the University of Tokyo Graduate School of Agriculture and his veterinarian license in
1983, and earning a Ph.D. in Agriculture in 1991.
He has worked at the National Institute of Health in Japan, the NERC Institute of Virology &
Environmental Microbiology in Oxford, UK, and as Director of the Department of Veterinary
Science at Japan’s National Institute of Infectious Diseases before assuming his current
position in 2019. He is mainly engaged in research on the risk and pathogenicity of zoonotic
diseases and vaccine development. He was a member of the WHO Advisory Committee on Variola
Virus Research from 2005 to 2011, a visiting professor at Gifu University Graduate School
from 2007 to 2019, and a cooperative professor at the University of Tokyo Graduate School of
Agriculture and Life Sciences from 2008 to 2019. He is also a member of the board of
directors of the Japanese Biological Safety Association and a member of the board of
trustees of the Japanese Society of Veterinary Science.